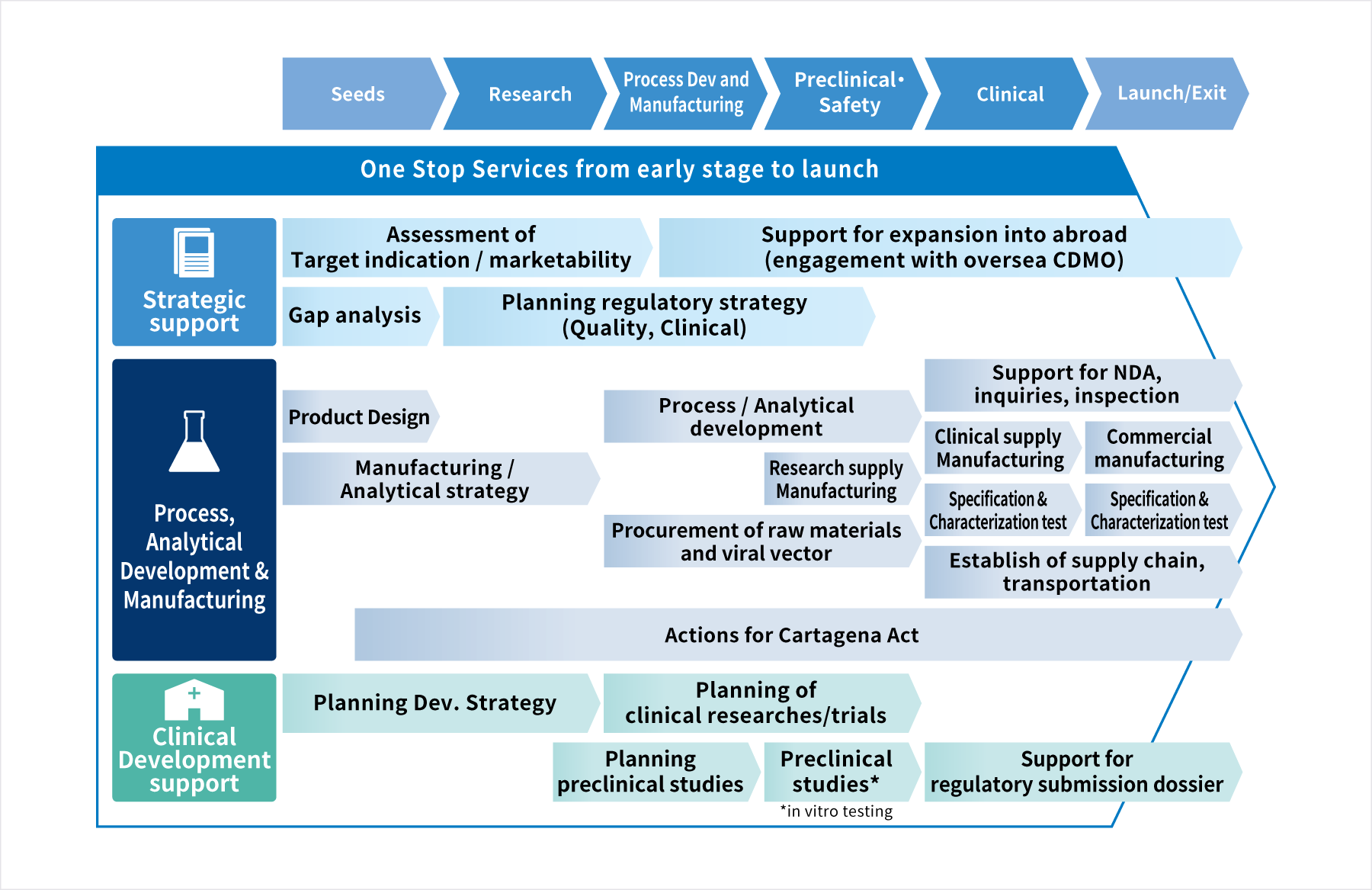
CDMO Service from Product Design to Commercial Production
- Development Consulting
-
- Building a development plan
- PMDA consultation
- Manufacturing of research supplies, clinical supplies, and commercial supplies
-
- Establishment and operation of manufacturing system
- Quality testing
- Design of Product Specification
-
- Setting quality specifications and safety of raw materials
- Development of manufacturing process
- Setting of specification
- Establishment of transportation process
The list of our services is below
- Service Menu List
-
R&D consulting, Issue identification, support of product specification design, etc.
Categories Menu overview Details 1) R&D Consulting Development Planning Support We support to build a development plan suitable for clients' technological seeds, including appropriate test items and their timing.
Our experienced consultants can support PMDA consultation from preparation.
In addition, we support you to comply with regulations in Japan, where overseas clients have high needs.Supports of PMDA consultation Consulting of regulation in Japan, for overseas clients 2) Identification of issues in manufacturing methods On-site assessment of manufacturing process/technology Our engineers check clients' process on site to identify issues in manufacturing methods and propose solutions. Assessment of manufacturing process/technology by co-trial (Carrying out clients' process together at our sites) 3) Design of product specification Support for setting quality specifications and safety of raw materials We support to select raw materials per product characteristics and propose quality specifications. For biological raw materials, we can support to confirm compliance with "Standards for biological raw materials". Support to develop manufacturing processes Based on product characteristics, we support the development of manufacturing processes from human cells/tissues to meet required quality specifications. Support for setting specifications From the viewpoint of quality and safety, we support process inspections, inspection items, and specification setting in the manufacturing process from the acceptance of source cells/tissues to the shipment of products. Support for setting up quality specification tests Support for building transportation system We support to develop packaging and carriers for transportation based on product characteristics as well as building of transportation systems to ensure quality and safety. Stability test support We support stability testing for quality and safety. Manufacturing process development Based on the product characteristics, we develop the appropriate process of manufacturing from source cells/tissues with meeting required quality specifications. Equivalence verification per changes in manufacturing methods We perform equivalence verification due to changes in manufacturing methods. 4) Manufacturing of preclinical supplies Manufacturing of preclinical supplies We manufacture the products for preclinical studies. Establishment and operation of production system Appropriate management of manufacturing and quality is required for the products of cell and gene therapy/regenerative medicine in accordance with the GCTP regulation in Japan. We build and operate an appropriate management system suitable for the client's product characteristics. Quality specification testing We perform required quality specification testing per specifications of products. 5) Manufacturing of clinical supplies Manufacturing of cell processing products Based on the procedure manual (SOP) from a client, we work on technology transfer then manufacture cell processing products. Establishment and operation of production system We build and operate an appropriate management system that is suitable for the product characteristics of each client’s product. Quality specification testing We perform required quality specification testing per specifications of products. 6) Improvement of manufacturing process Improvement of manufacturing process improvement for late-stage clinical supplies or commercial supplies We improve/customize the manufacturing process developed during product specification design for adjusting to late-stage clinical supplies and/or commercial supplies. 7) Manufacturing process development Development of manufacturing processes for late-stage clinical supplies or commercial supplies We develop the manufacturing process for late-stage clinical supplies and/or commercial supplies. Equivalence verification per changes in manufacturing methods We perform equivalence verification due to changes in manufacturing methods. 8) Manufacturing of clinical supplies Manufacturing of clinical supplies We manufacture clinical trial materials. Establishment and operation of production system for clinical supplies Appropriate management of manufacturing and quality is required for the products of cell and gene therapy/regenerative medicine in accordance with the GCTP regulation in Japan. We build and operate an appropriate management system suitable for the client's product characteristics. Quality specification testing We perform required quality specification testing per specifications of products. Support for building logistics of transportations Since products in cell & gene therapy/regenerative medicine require rigorous temperature control due to its limited shelf-life, it is necessary to optimize the transportation system for medical institutions per each product. Through the collaboration with the logistics companies who we partner with, we can support to deliver your products to your designated destination. Cell bank establishment and storage We support to establish cell banks for manufacturing, which are especially essential for allogeneic cell products, and conduct quality inspections such as microbial tests and characterization tests. The established cell bank is stored at an appropriate temperature under our management system. 9) Supports of works for regulatory submissions Data collections required for regulatory submissions We support and execute documentation for PMDA consultation and other regulatory interactions. Support documentation for regulatory submissions Works to regulatory inspections 10) Manufacturing of commercial supplies Technology Transfer We perform technology transfer including your technology, procedures, experiences and other important data to ensure successful manufacturing. Validation Execution We execute verification and validation of scientific rationale to ensure stable and sustainable manufacturing of your products. Manufacturing of approved products We manufacture your approved products. Quality specification testing for approved products We perform required quality specification testing per specifications of your approved products. Establishment and operation of a production system in accordance with GCTP regulation Appropriate management of manufacturing and quality is required for the products of cell and gene therapy/regenerative medicine in accordance with the GCTP regulation in Japan. We build and operate an appropriate management system suitable for the client's product characteristics. Shipment and logistics of approved products We ship and transport your approved products from our manufacturing sites. Stability testing We support stability testing to ensure quality and safety. 11) Process improvement of commercial manufacturing Process improvement of commercial manufacturing for approved products We work on the improvement of the manufacturing process of the your approved products. - Vector manufacturing and tests that can be handled by TFBS Bioscience, Inc.
-
EX: Phamacology testing, toxicology testing, characterization testing of cell banks
* TEIJIN group partners with TFBS Bioscience and they conduct the tests.
* These are the links to TFBS Bioscience web site.
Categories Sub categories Test Items/Contents Pharmacology Tests In vivo testing Animal species: rats, mice, guinea pigs, hamsters Route of administration:
Intravenous, intra-arterial, intracranial, intratracheal, subcutaneous, customized, intraperitoneal, intra-articular, intranasal, intracerebral, oral, intramuscularDisease Model: - • Respiratory system (Chronic obstructive pulmonary disease (COPD))/Rhinitis/ Asthma)
- • Acute liver failure (ALF)/Inflammatory bowel disease (IBD)
- • Acute/chronic kidney disease (AKD/CKD)
- • Diabetes
- • Skin care (whitening effect/moisturizing effect/Hair regeneration effect)
- • Cardiovascular
- • Solid tumors
- • Custom models
Toxicity Tests (Safety) General Toxicity Tests Bio-distribution test (GLP/non-GLP) Genotoxicity Tests Local hypersensitivity test Tumorigenicity Tests Pilot toxicity studies (non-GLP) Pivotal Toxicity Test (GLP) Bioanalysis for animal testing Clinical Observation Histopathology (IHC, H&E) Cell-mediated analysis * Primary cell culture for immunoassays Serology Analysis * Cell-based platform Titration of neutralizing antibodies * ELISA platform Cell Bank Characterization (Mammals) Identity test Karyotyping DNA Fingerprinting RAPD (Random Amplified Polymorphic DNA) Purity Tests Electron microscopy Sterility Tests Mycoplasma test Specific Virus Tests Virus testing (in vivo/in vitro) Retrovirus testing Mycobacterium test Stability Tests Nucleotide sequence analysis Genetic Stability Tests Other Exams Oncogenic Carcinogenicity Cell bank characterization (bacteria and yeast) - Viable bacteria count test Identification testing according to API 20E Bacteriophage testing Gram staining Direct culture method RAPD (Random Amplified Polymorphic DNA) Antibiotic Resistance Tests Sequence Analysis Restriction enzyme mapping and copy number analysis Confirmation of retention of expression constructs Virus Clearance Testing - - Bulk Rot Release Test - - Customized assay development and analysis for preclinical and clinical Mr./Ms. - - - Quality tests supported by TEIJIN Group
-
EX: Analytical development, cell morphology test and other confirmatory testing, stability testing
Test items Exam content Test method development Development of test methods in the early stages of development Confirmation test Cell morphology, appearance, properties, insoluble foreign matter test, differentiation capacity (osteoblasts, adipocytes, chondrocytes) Tests related to infectious agents Sterility test, endotoxin test, mycoplasma negation test, human virus negation test Safety Cell proliferation analysis Purity Tests Impurity test derived from manufacturing processes (cell purity test, albumin residue test derived from fetal bovine serum), immunophenotype (target cells, non-target cells), transgene copy number Impurities derived from the manufacturing process Proliferative retrovirus, residual viral vector, bovine serum albumin General Exams Appearance, Properties, and Insoluble Foreign Matter Tests Content test Viral copy number test, number of transgenic cells, viability test Characterization Gene transfer copy number test, cell surface marker test Biological activity or titer test Cytotoxic activity, cytokine production, gene expression Storage Stability Test Long-term storage test, transport stability test, post-freeze-thaw (use) stability test
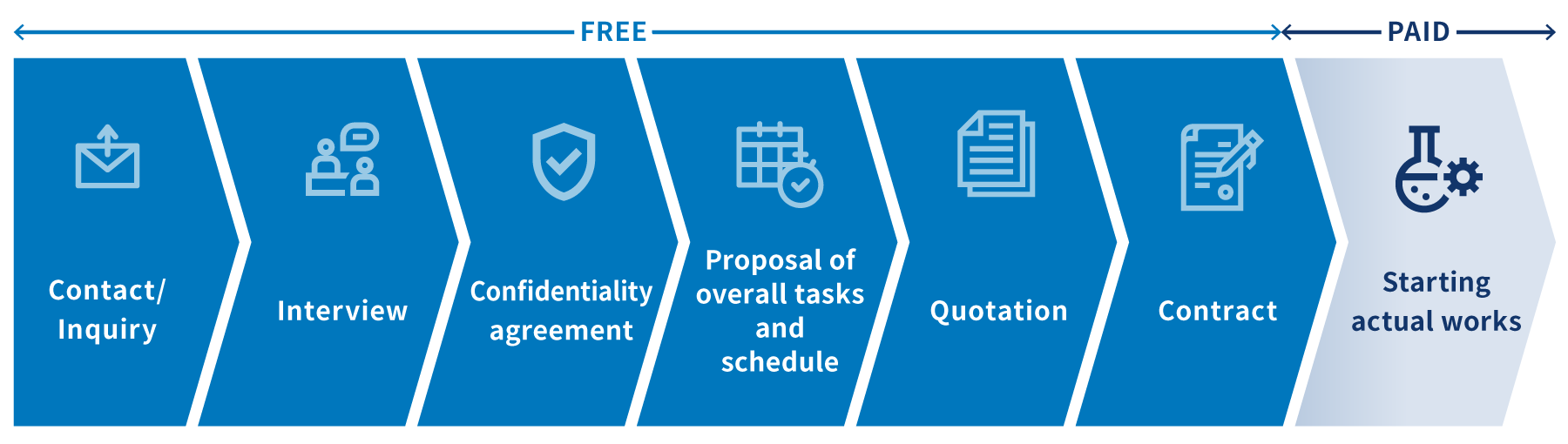
Inquiry flow
This is a general process from initial contact to actual works.