About Us
Regenerative medicine business of Teijin Group
Teijin Group's purpose is "Pioneering solutions together for a healthy planet", and we will achieve our Long-Term Vision: "To be a company that supports the society of the future" by being a company that prioritizes the health of the planet, protects the environment, and supports a circular society, as well as resolve issues for patients, families, and communities in need of greater support.
In accordance with the commitment, TEIJIN group offers Contract Development and Manufacturing Organization (CDMO) services through the group companies “TEIJIN REGENET Co., Ltd.” and “Japan Tissue Engineering. Co., Ltd. (J-TEC)”. TEIJIN REGENET and J-TEC specialize in cell and gene therapies including tissue-engineered products, with the aim of supporting the process development and manufacturing of cell and gene therapy products in Japan - taking innovative science from concept to patient treatment.



- TEIJIN REGENET Co., Ltd.
-
TEIJIN REGENET is established through an incorporation-type company split, with succeeding the rights and obligations arising from its CDMO business from Teijin. This company split enables agile and flexible operation of CDMO business. Through the license agreement between Teijin and J-TEC executed on April 2023, TEIJIN REGENET offers well established CDMO services with clients by utilizing J-TEC's expertise and know-hows.


- Japan Tissue Engineering Co., Ltd.
-
Japan Tissue Engineering Co., Ltd. (J-TEC) is a regenerative medicine manufacturer with the vision of "Creating a Future for Regenerative Medicine" and has been a member of the Teijin Group since March 2021. As a pioneer of regenerative medicine in Japan, J-TEC has obtained marketing authorization of the following regenerative medical products.
- October, 2007 : Autologous cultured epidermis "JACE ®" (Japan's first regenerative medicine product)
- July, 2012 : Autologous cultured cartilage "JACC ®"
- March, 2020 : Autologous cultured corneal epithelium "NEPIC ®"
- June, 2021 : Autologous cultured oral mucosal epithelium "OCURAL ®"
- March, 2023 : Autologous cultured epidermis containing melanocyte "JACEMIN ®"
Of the 20 regenerative medicine products approved in Japan, five are J-TEC products (as of April 2024).
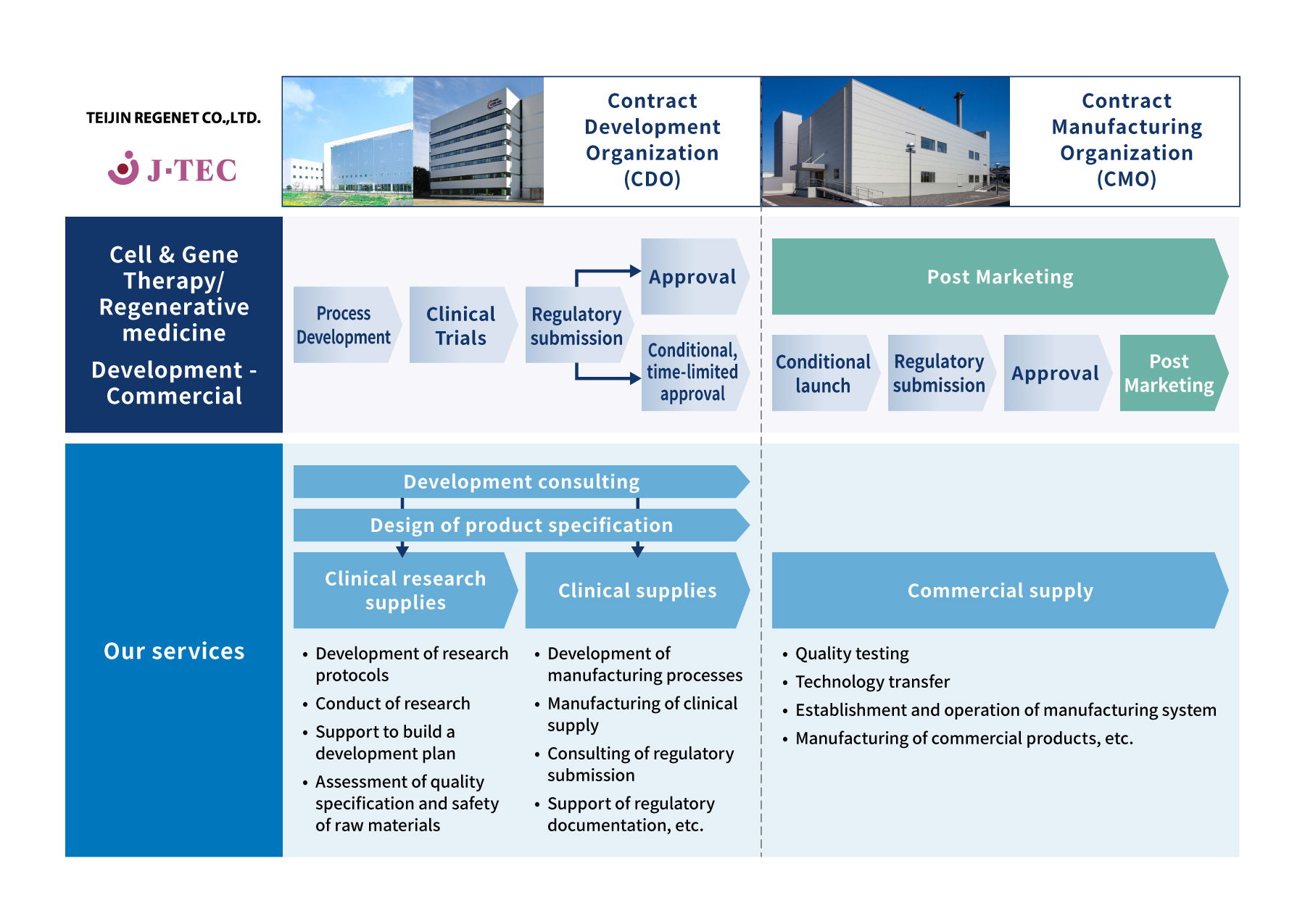
TEIJIN CDMO's strength
One-stop solution to realize the seeds of cell & gene therapy/regenerative medicine
"Kashiwa-no-ha Facility", CDO site of TEIJIN REGENET
- "Kashiwa-no-ha Facility" serves as a core manufacturing function in Kashiwa-no-ha regenerative medicine platform in collaboration with National Cancer Center, Mitsui-Fudosan, and J-TEC. This platform provides one-stop service from early research and development to manufacturing of clinical supply and business strategy with seed holders of cell and gene therapy/regenerative medicine, so that we can contribute to producing innovative medical solutions for the diseases with unmet medical needs.
- The site is adjacent to the National Cancer Center East Hospital and it enables to provide geographical and functional benefits for the R&D consortium.
"Iwakuni Factory", CMO site of TEIJIN REGENET
- "Iwakuni Factrory" provide the integrated manufacturing services through the seamless linkage with Kashiwa-no-ha Facility, from scale-up/scale-out of clinical supply manufacturing to commercial supply manufacturing. We can support various needs of manufacturing such as gene transduction, mass-scale cell culture, and tissue culture.
- We TEIJIN have experience in developing unique pharmaceuticals and medical devices in-house, establishing high-quality production technologies, and commercial supply chain in Japan and overseas. We provide commercial manufacturing services by fully utilizing well established know-hows.
Regulatory approval of the five regenerative medicine products, the largest number in Japan (4 products are on the market) *As of April 2024
- As a pioneer in regenerative medicine, we have cultivated our capability of development, production, and sales, and established the mutual trust relationships of with the regulatory authorities in Japan through the approvals of the multiple products.
- Of the 20 regenerative medicine products in Japan, J-TEC has been making 5 regulatory approvals, including the first and second regenerative medical products in Japan. This is the highest number of achievements of regulatory approvals (of 4 are launched) in Japan.
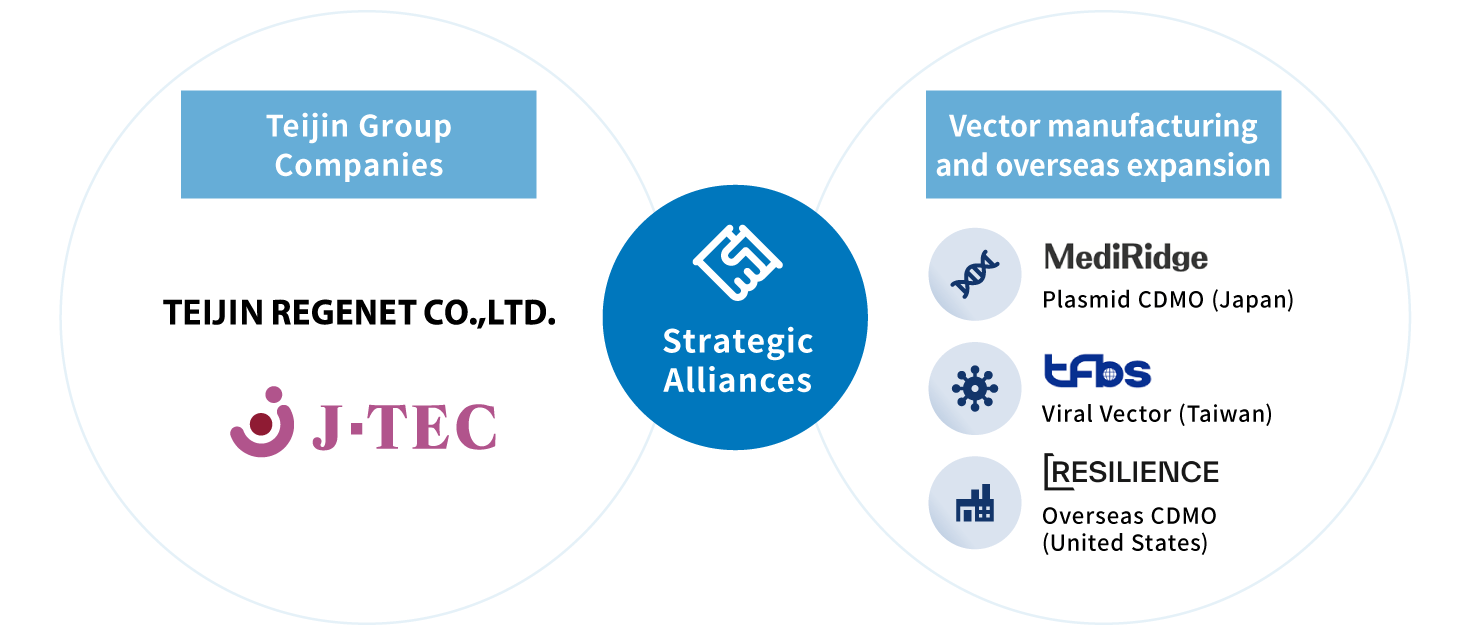
Supports of vector manufacturing and overseas clinical trials
- Through business alliances with TFBS Bioscience, Inc. (Taiwan) and Mediridge Co., Ltd.(Japan), we support viral vector manufacturing, supply, and related testing services. This collaboration enables to accelerate development of ex vivo gene therapy products.
- Through the alliance with National RESILIENCE, Inc. (United States), we can utilize their global manufacturing platform and support manufacturing of the products for global market expansion.
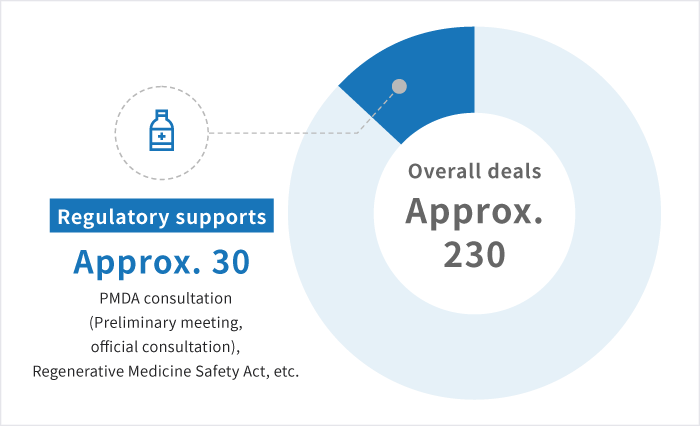
A track record of more than 230 deals *J-TEC results as of March 2024
We have experience more than 230 deals (number of the deals) in 5 years, mainly in academia and bio-ventures in Japan.
Our Manufacturing Expertise in various types of cells and vectors
(Below are representative examples)
Immune cells
- T-cells (CAR-T cells, etc.)
- Dendritic cells
- Lymphocytes
- Monocytes
Stem cells
- Mesenchymal stem cells (derived from bone marrow, adipose tissue, and synovium)
- iPS cells
(iPS cell-derived retinal pigment epithelial cells, iPS cell-derived intestinal epithelial cells, etc.)
Somatic cells
- Epithelial cells
- Pigment cells
- Chondrocytes
Vectors
- Lentiviruses
- Retroviruses
- Plasmids

